Thanks go to three of my clients who just this week asked questions about alkaline diet foods and pH:
What the pH?
Skin care commercials tell us their product will balance our skin, so-called “clean eating” blogs promote “alkaline diet foods”, designer bottled water companies and aggressive multi-level-marketing water filter companies promote alkaline waters, or maybe you retained a basic understanding of pH from biology or chemistry. The idea of an alkaline pH is trendy and in large print on labels.
But what does being “pH balanced” or “too acidic” or “alkaline” or even “too alkaline” really mean?
And, why does pH matter?
Because every enzyme, cell process, hormone, and system works best in a very defined level of acidity or alkalinity:
- Cells divide and replace themselves with new healthy cells at a correct rate only when the cell’s internal pH is correct—the same for healthy bacteria in various gut regions.
- Inside each cell, enzymes that digest foods, manufacture hormones, and eliminate toxic waste, do so only when the pH is correct.
- Outside each cell, hormones bind and give instructions only when the pH is correct.
- And in some cases, inappropriate cells like cancer make the environment outside itself quite acidic—they can grow, your healthy cells cannot.
What pH is correct? The great debate
If you “google” this subject, you’ll find controversy:
Argument #1: Our bodies are equipped to counterbalance a certain amount of acidity. In other words: you can’t change the pH of your body no matter what you do because it automatically self-corrects. (True, within reason)
Argument #2: Sure, but to self-correct the body needs to pull its resources away from what it should be doing. Including and especially digesting food, releasing minerals and key vitamins from food, keeping bones strong, balanced immune function, and fighting cancer. (Partly true. Especially if too many stressors and not enough genuine building blocks)
Argument #3: There is almost no actual research to either support or disprove these ideas. (False)
Think for yourself
Clients often ask, “When it comes to health, what role does pH really play?” or “I bought a [brand name] alkaline water unt that can bring the pH to 9.0, that’s good right?”
Is the cure for all health woes an alkaline pH? Everywhere? When considering a person’s health, we can’t consider pH in isolation. Doing so is too simplistic an approach—one that fails to take into account the complex role pH plays in our bodies and the different acidity (low pH) or alkalinity (high pH) in different places within our body.
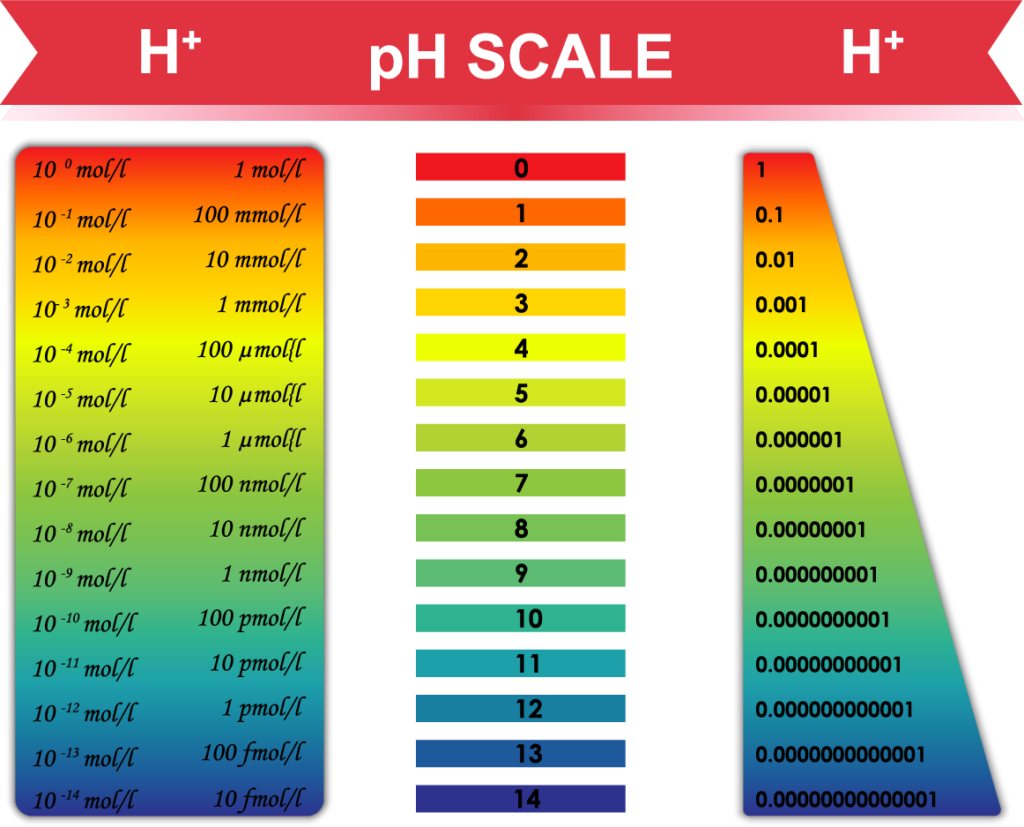
First and foremost, understand the pH scale
Warning: Basic chemistry ahead—not difficult, take it slowly if this is unfamiliar.
Ignore the colors. pH is in the numbers:
The pH scale measures acidity and alkalinity in terms of the amount of hydrogen ions (acid = H+) or hydroxyl (alkaline = OH-) ions. Water is formed by combining the two in equal amounts. After combining all available H+ with OH- to make water, if there are leftovers this determines the pH of that substance.
In other words, if there are equal amounts of H+ and OH- ions, the material has a perfectly neutral pH of 7.0. If more H+ than OH- ions, then the material is acid and the measured pH is less than 7.0. The decimal point matters.
On a measuring device or pH paper strip, readings less than 7.0 are acidic (with 0 being the most acidic—car battery acid is ~2.0 pH) and readings above 7.0 are alkaline (with 14 being the most alkaline).
Important point and why the decimals matter: Each whole number change in pH is a 1000x increase or decrease in hydrogen (H+). Biologically speaking, 1000x is a huge change and can make or break whether a cell can live or an enzyme can work.
For example, self-regulation of blood pH is one of the most carefully controlled systems in the human body; it is carefully maintained within a very narrow range of pH 7.35 and pH 7.45, a difference of a mere 100 H+ ions. In other words, most of us run at a blood pH of 7.4 which is slightly alkaline. That difference of just 100 H+ in blood makes a huge difference to your body.
Different body parts and systems should have a different pH
Inside your body, different areas have different amounts of acidity (or pH). There is no one “correct” reading for the entire body.
Skin
- Your skin is healthiest at a pH of 5.5 (acidic). Your skin needs to be slightly acidic to deal with environmental factors like bacteria and other toxins.
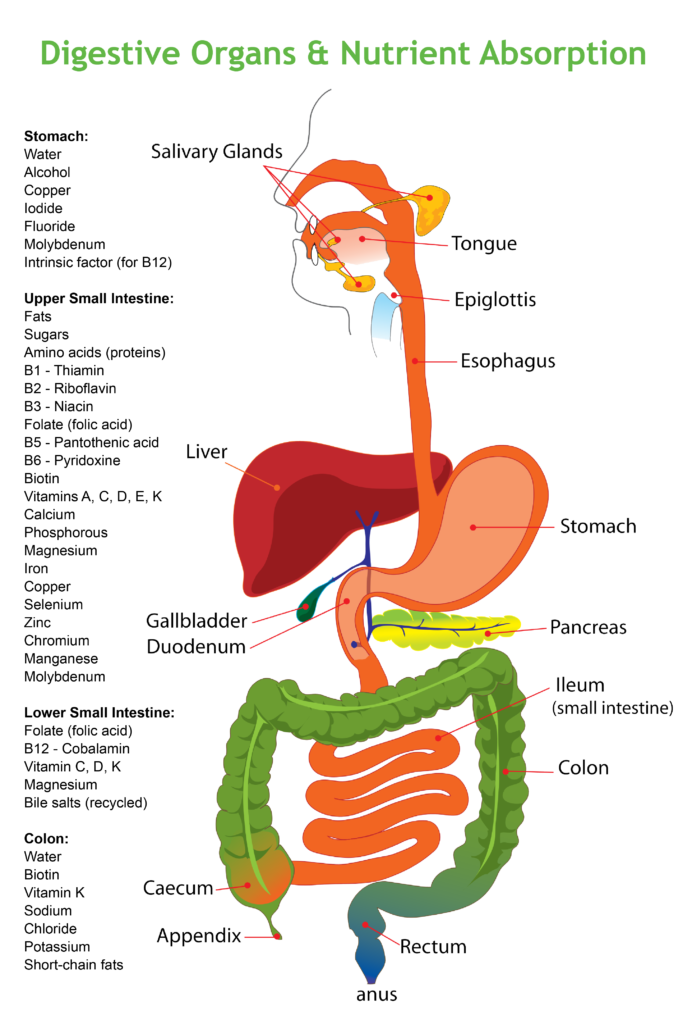
Digestion
- Digestion starts in the mouth. Saliva has an ideal pH of 7.4 (slightly alkaline, like blood) where enzymes that digest carbohydrates can work.
- By the time (hopefull chewed to mix in salivary enzymes) reaches your stomach, pH drops from between 5.0 and 6.0 (your stomach’s non-food resting state) to below pH 2.0. In young and healthy adults it takes about 45 minutes before enough acid is generated to lower the pH to 1.5-2.0. High stomach acid (very low pH) is a must for releasing minerals from food and certain vitamins, especially B12. The harsh pH 2.0 or less (acidic) environment of the stomach also serves to kill unwanted bacteria, parasites, and neutralize viruses.
- Doctors worried about heartburn or GERD (gastroesophageal reflux disorder) grab esophageal and gastric pH meters then decide based on that one test point whether or not to recommend antacids. See above on why stomach acid is necessary.
- When stomach pH is low enough (below 2.0), the partly-digested food is released into the small intestine where it is met by pancreatic and liver/gall bladder enzymes that bring the pH up to pH 6.
- As the food moves through the small intestine, pH continues to rise to pH 7.4 before the small intestine joins the large intestine (colon).
- In the colon, pH drops again to 5.7 at the juncture of the small and large intestine and then rises to nearly 7.0 towards your rectum.
Each section of the digestive system varies in pH to carry out its functions at that time. This is all a biological necessity to make proteins, fats, minerals, and vitamins, available for release and absorption.
Kidneys, bladder, and urine
Urine is made up of water, salts, and waste products from the kidneys. Measuring urine pH can give a sense of renal function. The pH in the nephrons of the kidney determines whether waste products and minerals are eliminated or reabsorbed—and… the nature of those waste products can influence urine pH. Ideally, the pH of urine should be around 6.0 to 7.5 but your doctor may not raise an eyebrow unless urine pH drops below 5.5, the cutoff for calling urine acidic.
When urine pH drops (acidic), this can lead to various forms of kidney disease, lower filtration rate with higher toxic waste reabsorption including reabsorption and retention of uric acid (gout).
The bottom line: Because different parts of our bodies serve different purposes. Each of these purposes and their related processes requires a particular acid–alkaline environment for optimum function.
Alkaline diet foods and lifestyle can keep each organ optimally acid or alkaline for its best performance
Most practitioners and home testing instructions check the pH of blood, saliva, and/or urine—substances we can test easily. This has led to the mistaken belief that pH levels remain the same throughout the body, at all times, when in fact nothing could be further from the truth.
The mistake lies in testing them once. In order to get the full picture of your body’s pH, these tests need to be done repeatedly. Not just once on one day, but over a period of time to provide a window into what is going on in your internal world.
Balance and maintain the pH level of your body to promote better health and well-being.
I fully agree that most people could benefit by addressing their acid–base balance, but before anyone begins megadosing on supplements or downing gallons of alkaline water, let’s understand what is really happening, why that may be important, and what to do (or not do).
The confusion and controversy: Enthusiasts of the “pH miracle” say that living in the modern world—with its reliance on refined grains and sugars, corn-fed beef, and unhealthy fats—means we are all overly acidic. This is true—but exactly where are we overly acidic?
These enthusiasts claim that to rectify this, we should focus all our attention on restoring “healthy blood pH” of 7.35–7.45. Hmmmm… isn’t that range already tightly maintained?
For now we see through a glass, darkly. —First epistle, Chapter 13, Corinthians
The clarity of the situation is obscured.
Since blood pH is the most tightly regulated of all the body’s systems, just what does it take to change blood pH? And, in attempting this, why would anyone ignore the changes occurring in other cells, organs, and systems?
Your body will protect blood pH and keep it within the 7.35 and 7.45 range at the expense of all other systems. To do this, your body will leach minerals, especially calcium, from your bones and other places.
With so many variations on pH in different parts of the body, why focus on blood anyway?
The concept of alkalinizing “the body”, is flawed
First off, what causes too much acid? that depends on where you measure it.
In the stomach, a large meal or heightened stress slows digestion:
- Large meals require substantially more stomach acid production to reach the very low pH needed by the stomach. Large meals stay longer in the stomach, can begin to produce gasses (ferment) and increase pressure within the stomach that causes reflux back into the esophagus.
- If we try to consume our meals on the run, while arguing, while under any stress at all, we shift into “fight or flight” and prevent the “rest, heal, repair, and digest” mode that allows the digestive tract to work well along its entire length.
The small intestine might arrive at an inappropriate (for it) pH due to:
- An imbalanced microbiome, especially SIBO where bacteria (or yeast—SIFO) have moved from the large intestine into the small intestine due to an inviting environment for them. What’s an “inviting environment”? One that fosters them. Invasive yeast and certain unhealthy bacteria tend to like a more acidic environment. The colon is slightly more acidic than the small intestine. If the small intestine pH remains lower (more acidic) than ideal, colonic microbes (that are appropriate for the colon) may move into the small intestine where they do not belong and produce gas from the food you have just consumed (bloating and pain).
- Non-optimal liver-gall bladder and/or pancreatic function as these organs provide a mix of enzymes and more that neutralize the high acidity of the stomach.
- Diets high in refined sugars and starches, and red meat.
Inappropriate urine pH
- Top on the list is diet. studies over many decades on large numbers of people (over 22,000) show that diets high in vegetables and some fruits (alkalinizing foods) while lower in grains (especially refined), sugar (especially refined), dairy, and red meat (acidifying foods) affect urine pH—and therefore the pH in your bladder and kidneys.
- Diarrhea causes a loss of electrolytes thus lowering both blood pH and colon pH.
- Use of diuretics make the urine more acidic.
- Diabetes and/or pre-diabetes cause a sequence of less ammonia production by the kidneys resulting in acidic urine. Interestingly, whether diagnosed or not, a study of 1000 individuals found that those with urine pH at or below 5.5 had 2.5 times more risk of developing diabetes than those whose urine pH was above 6.5.
Any factor that lowers urine pH (acidic) causes the kidneys to work extra hard to eliminate the excess H+ ions (acid).
How to improve pH balance?
Myth: Drink highly alkalinized water? What’s the hype?
Natural, uncontaminated spring water that has filtered through layers of earth averages ph 7.4—mildly alkaline. For eons, humans have consumed clean, spring water that contains many healthy minerals provided by planet Earth. Our bodies, systems, and cells depend on the minerals in clean, spring water.
Water contaminated by air pollution or any pollution is more acidic, think “acid rain” whether from ground runoff or other. pH is not a regulated water quality, just an indication that there may be contaminants. It is up to the municipality to choose to treat tap water for its pH. Your liver and kidneys (and skin) work extra hard to remove these contaminants.
By comparison, distilled and everse osmosis waters tend to range between pH 5 and 7—acidic. They also lack important minerals like calcium, magnesium, and trace minerals and will pull these out of your body.
The new trend: Alkaline water; usually pH 8 to 9. Despite health claims made by high pH, highly alkaline water, there is no scientific evidence that drinking water at this pH is beneficial. What is more likely is that the filtreation units remove contaminants; thus reducing the need for the body to eliminate them. As for commercial alkaline waters, bottling companies are not required to test the pH so this may or may not be what you are buying.
Interestingly, there is a small 2012 study asking whether alkaline water can benefit acid reflux. Damage to both the larynx and the esophagus due to different forms of reflux is due to having inactive pepsin—which needs an acid environment to work. Alkaline water instantly and permanently inactivates pepsin. That doesn’t mean the stomach produces less acid, it probably must produce more to reverse the effect of the alkaline water, inactivated pepsin at high pH can not be reactivated.
More on pepsin…
Pepsin is an enzyme that primarily digests meat. People who don’t like meat often have digestion difficulties. There are some nutrients we can get only from meat.
With an alkaline diet food plan, your choices should balance acid-producing with alkalinizing and err on the side of alkaline food
(download this chart here or by clicking on the image)
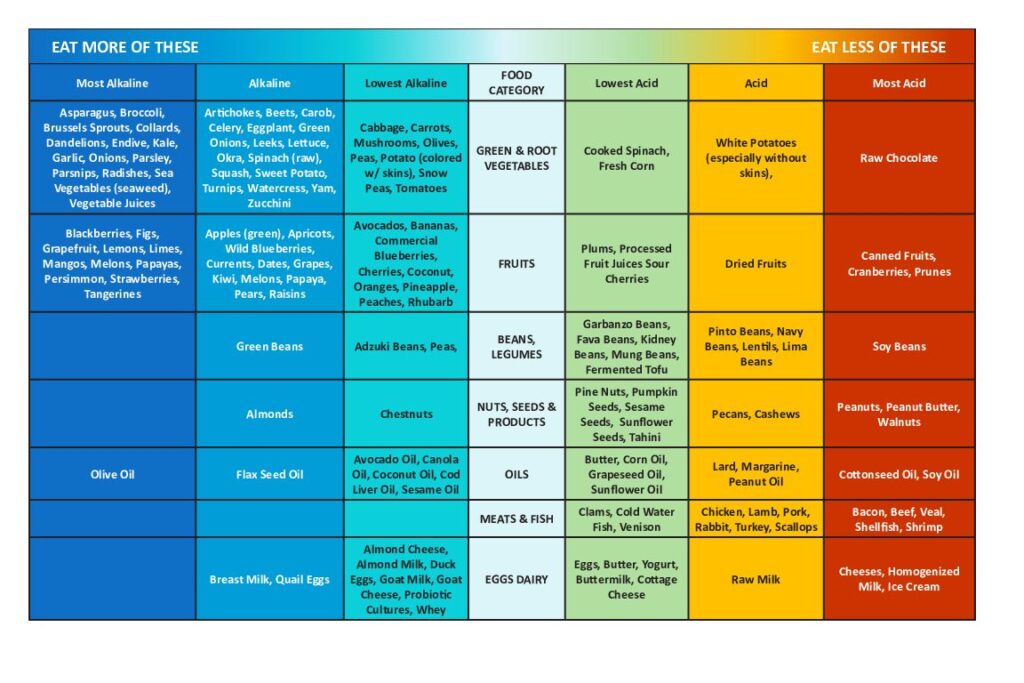 Whether a food is acidifying or alkalizing can require some mind-bending, because some foods that we think of as “acidic” are, in fact, alkalizing, like lemons for example.
Whether a food is acidifying or alkalizing can require some mind-bending, because some foods that we think of as “acidic” are, in fact, alkalizing, like lemons for example.
We might normally think of grains and milk as meek and mild in nature but these are acid-forming when ingested.
The pH of the food as it goes into our bodies isn’t important; what is important is the resultant pH once the food is broken down.
Acid isn’t all bad… We need protein: but it is acidifying… balance
Proteins—especially red meats but also plant-based proteins—need huge amounts of alkaline minerals for complete digestive processing. When you eat meat or plant-based proteins, your system creates an acid load. To balance this, it goes looking for alkaline minerals currently in the digestive tract, usually from plants. If it fails to find alkaline nourishment there, it draws on the calcium, magnesium, phosphorus and potassium minerals stored in our bones.
pH and disease
Dietary plants and then bones are the storehouse for alkalizing minerals. When the body has to offset acid overload in the digestive tract by pulling minerals from bones, we lose bone density which can lead to osteopenia and ultimately osteoporosis.
But bone loss is only one health issue that stems from failing to balance acidifying foods with alkalinizing ones.
Over time, attempting to neutralize an acidifying grain, sugar, processed foods diet—or even a diet high in protein that doesn’t also include lots of alkalinizing vegetables—will imbalance your body in a way the begins to elevate the pro-inflammatory blood acid homocysteine. Studies show that high levels of homocysteine in the blood double the risk of osteoporosis–related fractures, along with other inflammatory conditions like heart attack, stroke, fuzzy thinking, and Alzheimer’s disease.
Naturally improving your pH balance
 Ideas to restore pH balance, support healthy digestion, keep blood pH levels on track, and protect your bones and kidneys, too:
Ideas to restore pH balance, support healthy digestion, keep blood pH levels on track, and protect your bones and kidneys, too:
Skip the processed foods. Replace convenience foods with alkalizing plants and other foods.
Address and fix all sources of acidity: Help your body remove toxic chemicals which takes place more slowly in an overly acidic environment. How?
- Fill your plate with fresh vegetables, particularly the dark green leafy kind. Eat the rainbow: foods that are fresh, organic, and deeply pigmented or brightly colored are the kinds that benefit you the most!
- Add fresh lemon or lime juice to foods and beverages as a highly alkalizing flavor accent. Try this easy electrolyte drink recipe that is soooo much better for you than Gatorade.
- Choose root vegetables, too. Root veggies are an excellent sources of alkalizing mineral compounds. Roasted Root Veggies, which are also high in inulin—a type of prebiotic or food for friendly gut flora—paves the way for beneficial bacteria to thrive further down into the colon. These helpful bacteria help digest foods and release minerals and other alkaline materials.
- Have plenty of “green foods” and “green drinks“. These contain the pigment chlorophyll, the plant world’s equivalent of the hemoglobin in our blood. Chlorophyll is a powerful detoxifier, builds your immune system, and provides magnesium.
- Eat plenty of protein as protein builds strength and provides energy. Because all proteins are acidifying, eat them with plenty of veggies. Grains and starches are also acidifying; that’s where the Standard American Diet gets into trouble: burgers and fries, chicken and rice, turkey sandwiches… even whole grains.
- Eliminate all processed foods, particularly those that contain partially hydrogenated oils (trans fats).
- Clear the digestive slate with a the Standard Process 21-day Purification Program. This program starts by resting your digestive tract, then restores gut health and balance, and finally gives you tools to better understand which foods may be irritating your health.
pH is just the tip of the iceberg. Sure, it is good to pay attention to changes you can make to improve balance. Paying attention to your pH includes steps that make an immediate positive change towards long-term health in other ways. Just, please, don’t get caught up in expensive gimmicks.
Continue to listen to your body and help it find balance on all fronts, including your hormones, your emotions, and your lifestyle.
References
Fallingborg J. (1999) Intraluminal pH of the human gastrointestinal tract. Danish medical bulletin 46(3):183-96
Fenton, Tanis R, and Tian Huang. (2016) Systematic Review of the Association between Dietary Acid Load, Alkaline Water and Cancer. BMJ Open 6.6
Koufman, J. A., & Johnston, N. (2012). Potential benefits of pH 8.8 alkaline drinking water as an adjunct in the treatment of reflux disease. The Annals of otology, rhinology, and laryngology, 121(7), 431–434.
Naude MTech Hom D. F. (2022). Chronic Sub-Clinical Systemic Metabolic Acidosis – A Review with Implications for Clinical Practice. Journal of evidence-based integrative medicine, 27, 2515690X221142352.
Storz, M. A., & Ronco, A. L. (2023). How Well Do Low-PRAL Diets Fare in Comparison to the 2020-2025 Dietary Guidelines for Americans? Healthcare (Basel, Switzerland), 11(2), 180